Tumor Biology and Microenvironment Theme 3 Accomplishments
Our investigators study the interaction of tumor cells with mesenchyme and inmate immune cells. We also identify, evaluate and utilize cellular and humoral immune mechanisms to develop immunotherapy. As summarized in Figure 6, taking advantage of our active bone marrow transplant group and the cGMP facility, our clinical studies in Theme 3 made significant progress in improving clinical outcomes via activated armed T cells or DNA vaccine. In the meantime, our preclinical studies continue to explore the underlying mechanisms of anti-tumor therapeutic agents and investigate how to block adverse pro-inflammatory host responses. Dr. A.Thakur showed that engagement of targeted T cells to tumor cells not only kills tumors cells and releases cyotkines and chemokines to shift the tumor microenvironment to a Th1 anti-tumor environment with consequent reduction of myeloid derived suppressor cells and decreases in the induction of T regulatory cells (Thakur, JTM, 2013, Thakur, CII, 2012).
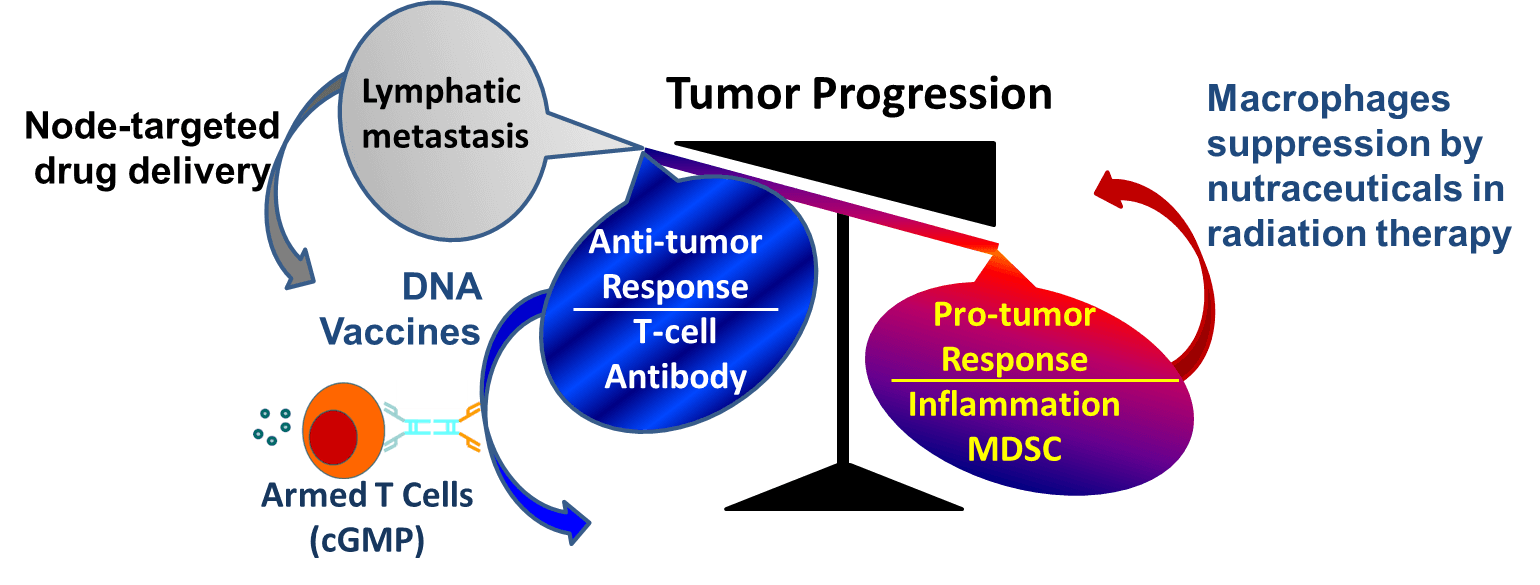
Highlights of the clinical and preclinical studies in Theme 3
Our clinical members continued to initiate or participate in clinical and preclinical studies at KCI. Sixteen new clinical trials, ranging from Phase I to Phase III, were initiated to test or validate new drugs or treatment protocols. The bone marrow transplant group continued to lead in clinical trials. In an effort to further explore the therapeutic potential of bispecific armed active T cells, the laboratory showed that EGFR-bispecific armed active T cells were highly toxic against multiple glioblastoma cell lines (Zitron IA, BMC Cancer, 2013). In addition, pretreatment of tumor cells with Pan-Bcl-2 inhibitor AT-101 enhanced tumor cell killing by EGFR targeted T cells (ref). Dr. M Abidi received the sponsorship from Multiple Myeloma Res. Foundation and Onyx Pharmaceutical to conduct a Phase Ib/II trial of oprozomib and Dexamethasone and a phase I study of ganetespib, respectively, in patients with relapsed and/or refractory multiple myeloma. Dr. A Deol started Pharma-sponsored clinical studies of two new autologous stem cell transplantation protocol for hematologic malignancies, and participated in a Phase III study on the efficacy and tolerability of Budesonide in patients with resistant oral chronic GvHD. On the side of solid tumor treatment, Dr. M Cher participated in a Phase III randomized trial of autologous dendritic cell immunotherapy plus standard treatment of advanced renal cell carcinoma. Dr. U Vaishampayan continued to be highly active in clinical studies and started 4 more Pharma-sponsored Phase I studies of MDV3100, BKM-120 and other regimens in patients with metastatic prostate cancer, renal cancer and urothelial cancer. Dr. L Lum and M Yankelevich (pediatric oncology) showed that anti-CD3 x anti-GD2 bispecific antibody armed T cells were highly cytotoxic to neuroblastoma cell lines (Yankelevich, Pediatr Blood Cancer, 2012. Drs Lum and Yankelevich have a FDA-IND approved product for a phase I/II GD2 targeted T cells infusions in neuroblastoma patients in collaboration with Dr. NK Cheung at Memorial Sloan-Kettering Cancer Center. In addition, a Phase Ib study based on the results of Dr. W-Z Wei was initiated to establish the safety and optimal dose of the hybrid Her-2 DNA vaccine (pE2-NeuTM) in Her-2 positive metastatic tumor (ref).
Our new member Dr. H Liu, a pioneer in the field of subunit vaccines (made of protein or sugar fragments), recently developed a novel nanoparticle-based method to direct the hitchhike of vaccine molecule to lymph nodes (Ref). The procedure involves albumin which consists of "binding pockets" to catch fatty molecules that are specifically enriched in lymph nodes. In the initial experiment to prove the concept, Dr. H Liu and colleagues created a series of vaccines that targeted certain diseases, such as cervical cancer and melanoma, in mice and found that each vaccine made a large population of memory T cells that were specific to the viral or tumor peptide. Compared with the peptide antigens alone, the albumin-targeted vaccines triggered immune responses that were five to 10 times stronger.
Inflammation, from innate immune response to tumor response or side effects for radiation therapy, is known to promote tumor progression and comprise cancer treatment. In collaboration with Dr. S Gadgeel and other colleagues, Dr. G Hillman studies the effects of soy isoflavones to decrease or prevent the inflammation associated with lung cancer radiation therapy (ref), and showed that when given pre- and postradiation in an animal model, soy isoflavones protected the lungs against adverse effects of radiation including skin injury, hair loss, increased breathing rates, inflammation, pneumonitis and fibrosis. This study provided intriguing evidence for a radioprotective effect of soy, which is otherwise nontoxic. Currently, there is an investigator-initiated clinical trial at KCI to test whether soy isoflavones can radically improve the effectiveness of the radiation treatment for inoperable non-small cell lung cancer.